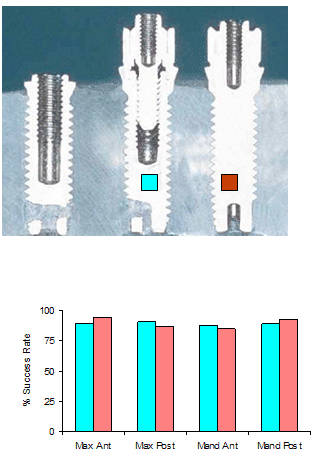
 Five Years Prospective Human Clinical Studies were conducted using the Hexagonal Abutment Implant System (HAIS). A comparative study of two vs one-stage type HAIS in human clinical trials was done with using 382 screw-type titanium implants in a two-stage surgery while 461 identical implants were placed in a one-stage surgery. The 3mm hexagonal abutment protruded above the oral mucosa during the three to six month healing period. A total of 172 consecutive patients over a 5-year period were evaluated. Clinical parameters such as pocket depth, gingival health, attached epithelium, plaque index, bone loss, calculus index, mobility and pain were evaluated in both techniques. Age, gender, implant length, jaw
locations, bone density and type were also analyzed. A five year life table (Kaplan-Meier) analysis indicated no significant difference in survival between the two-stage (91.6%) and the one-stage (89.7%) P>0.05. Five Years Prospective Human Clinical Studies were conducted using the Hexagonal Abutment Implant System (HAIS). A comparative study of two vs one-stage type HAIS in human clinical trials was done with using 382 screw-type titanium implants in a two-stage surgery while 461 identical implants were placed in a one-stage surgery. The 3mm hexagonal abutment protruded above the oral mucosa during the three to six month healing period. A total of 172 consecutive patients over a 5-year period were evaluated. Clinical parameters such as pocket depth, gingival health, attached epithelium, plaque index, bone loss, calculus index, mobility and pain were evaluated in both techniques. Age, gender, implant length, jaw
locations, bone density and type were also analyzed. A five year life table (Kaplan-Meier) analysis indicated no significant difference in survival between the two-stage (91.6%) and the one-stage (89.7%) P>0.05.
The hexagonal shaped abutment of the HAIS can be used in various clinical applications. Pullout tests were conducted to evaluate the retentive strength of cemented copings in unmodified and modified forms. The unmodified hexagonal abutment produced the highest retentive strength, removal of one of the hexagonal surfaces maintained 80% of the retentive strength. Further removal of the second and third adjacent surfaces produced no significant reduction in retentive strength. Removal of the remaining surfaces produced a cylindrical abutment with the weakest retentive strength. Clinically, selective removal of three adjacent surfaces on the hexagonal abutment allows its use in multiple implant bridge construction. The concept of using different abutments for different types of prosthetic applications
needs to be challenged. A standardized abutment can greatly simplify both treatment planning and prosthetic protocol in implant dentistry. The Hexagonal Abutment Implant System consists of only six components which has been successfully applied to restore all implant cases.
Supported in part by National Research Council of Canada # 28048U.
|