| Aboyoussef, Hoda; Weiner, Saul; Zweig, Barry; Thompson, Van: University of Medicine & Dentistry of New Jersey, 110 Bergen Street, University Heights, Newark, NJ, 07103, USA�Kwan, Norman; Yang, Silvia: Canadian Dental Implant Institute, 206 King Street, St. Catharines, ON, L2R 3J7, Canada� |
|
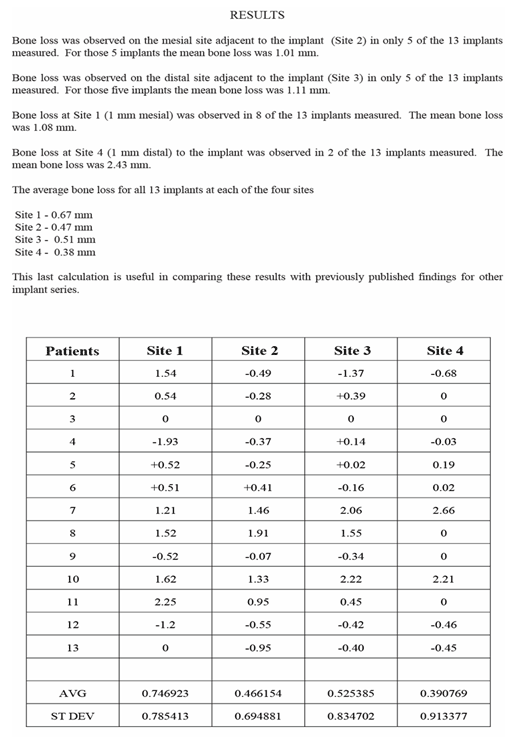 A one-piece abutment-implant system has the advantage of eliminating the abutment screw and simplifying restorative procedures. The one-piece device is designed such that a polished 3mm abutment protrudes at one end with a sandblasted, acid-etched surface screw implant at the other. A one-piece abutment-implant system has the advantage of eliminating the abutment screw and simplifying restorative procedures. The one-piece device is designed such that a polished 3mm abutment protrudes at one end with a sandblasted, acid-etched surface screw implant at the other.
In this study, 158 one-piece, non-submerged implants with a threaded, rough osteophillic surface and a polished 1mm collar were placed with the abutment platform flush with the crestal bone. The implants were restored after ten weeks. Radiographs were taken one month, two and one-half, six, nine, and twelve months post-insertion of the one-piece implants. Crestal bone loss was measured from the serial radiographs using digital analysis with AutoCAD2000 software. Vertical and horizontal changes in the crestal height were measured. Use of this software permits calculation of the volumetric loss as well.
After 12 months, the pattern of bone loss observed differed from that seen in conventional two-stage systems. No dieback to the first thread was observed. Loss of crestal bone from areas adjacent to the 1mm polished collar was observed (1.270mm, SD 0.759mm). However, in addition a horizontal crestal loss of 0.827mm, SD 0.539mm occurred. This horizontal crestal loss has not been reported with two-stage systems. Moreover, the amount of crestal loss (relative to the margin of the tooth supported crown) is less since no implant-abutment surface is present.
Use of a one-piece abutment-implant system, in addition to its technical simplicity for prosthetic restoration, may also be useful biologically to reduce the degree of crestal bone loss, and in keeping potential periimplant pocket depth to a minimum.
1. Buser D, Weber HP, Lang NP: Tissue Integrated on Non-Submerged Implants. One Year Results of a Prospective Study with 100 ITI Hollow-Screw and Hollow Cylinder Implants. Clinical Oral Implants Res 1990; 1:33-40.
2. Hermann JS, Cochran DL, Nummikoski PV, Buser D: Crestal Bone Changes Around Titanium Implants. A Radiographic Evaluation of Unloaded Nonsubmerged and Submerged Implants in the Canine Mandible. J Periodontol 1997;68:1117-1130.
This research is supported by Biomedical Implant Technology Inc.
|